Why metals corrode
All metals have a propensity to corrode due to their nature and manufacturing process. During manufacture (smelting) a great of energy in the form of heat is transferred to the metal.
During cooling, this has the effect of changing the micro-structure of the metal and creating microscopic areas of ‘difference’ within the metal. In iron, the micro-structure consists mainly of ferrite and pearlite.
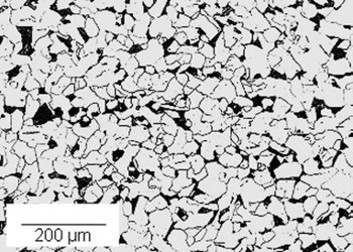
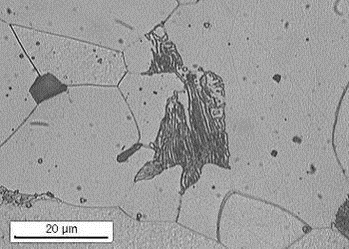
In the presence of an electrolyte reactions take place between the ferrite and the pearlite, these reactions result in wastage of the ferrite and the formation of rust at the sites of the pearlite. The sites of the ferrite are known in corrosion parlance as ‘anodic’ sites and the pearlite locations are known as ‘cathodic’ sites.
Metal corrosion always takes place at the anodes and corrosion products (rust) form on the cathodes. (See rust prevention)
The steel corrosion reaction
 Anodic Reaction:
Anodic Reaction:
Fe à Fe2++ 2e-
Cathodic Reaction:
½O2 + H2O + 2e- à 2OH-
Combined Reaction:
Fe2++ 2OH- à Fe (OH)2
Ferrous Hydroxide
Oxidises à Fe2O3(H2O)
Hydrated Ferric Oxide – Red Rust
The basic corrosion process:
2Fe2O3 + 3C → 4Fe + 3CO2
( Conversion to iron )
Fe + O2 + H2O → Fe2O3.H2O
(Change back to Ore-like state )
(Read more about cathodic protection)

Metal corrosion rates
Metals corrode at different rates; they can be ranked in an order of ‘corrodibility’.
Luigi Galvani, an Italian scientist, worked out the order of corrodibility of metals.
This ‘ranking’ of metals became known as the Galvanic Series in his honour. (Read more about galvanic corrosion.)
